What follows is a list of research that shows how a ketogenic diet can have effect on the immune system. A lot of this research is done in non-human bodies or circumstances so none of it has been proven to be similar in humans.
This is very important to know because mice and rats have a somewhat more different and stronger immune system than humans.
1. A first, most recent article is where it helps gamma-delta T-cells respond better to Influenza. How exactly the ketogenic diet caused an increase in these gd T-cells is not clear yet.
“Ketogenic diet activates protective γδ T cell responses against influenza virus infection” https://immunology.sciencemag.org/content/4/41/eaav2026
2. The next one talks about improved functioning of CD8-T cells. However, this is caused by the enhanced PGC-1α expression. Beta-hydroxybutyrate (BHB) and butyrate itself increase PGC-1α expression through their HDAC inhibitory function. But butyrate may have the upper hand in this.
“Enforced PGC-1α expression promotes CD8-T cell fitness, memory formation and antitumor immunity” https://www.nature.com/articles/s41423-020-0365-3.pdf
“Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule” https://www.nature.com/articles/s41598-018-36941-9
“Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3735349/
3. Further on the CD8-T cells we find epigenetic modulation. The result is that these cells are able to divert energy towards glycogen, which is the stored form of glucose. What I suspect from this is that it gives these T-cells the ability to respond more quickly, proliferate more quickly when a pathogen is detected. Just like cancer cells, fast growing cells need a lot of glycolysis, not only to support the metabolic demand but also to synthesise fatty acids to form membranes for the new cells. I see a potential link here with the first paper on gd T-cells where the same mechanism could help in the fast proliferative response.
“Ketogenesis-generated β-hydroxybutyrate is an epigenetic regulator of CD8+ T-cell memory development.” https://www.ncbi.nlm.nih.gov/pubmed/31871320
4. We continue a bit more on CD8-T cells. When they are challenged in an environment of low glucose and low oxygen, they switch over to fatty acid catabolism. In a tumor environment where cancer cells take up glucose 10x to 100x more than normal cells, glucose becomes scarce. Being able to switch fuel can help preserve efficacy. Hypoxia itself changes their metabolism towards fatty acids. If they are shown to take up BHB then it could be a very effective way to continue that efficiency against cancer. And the previous article seems to indicate this is the case.
“Enhancing CD8+ T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5751418/
5. The following study looked at BHB supplementation and noted increased INF gamma output from T1 helper cells.
“Acute hyperketonaemia alters T-cell-related cytokine gene expression within stimulated peripheral blood mononuclear cells following prolonged exercise.” https://www.ncbi.nlm.nih.gov/pubmed/31729600
6. Not only CD8 T-cells but also CD4 T-cells seem to be affected positively. The next paper fed mice high fat and saturated fat, thinking it would result in a negative situation. However, what they found was a reduction of cholesterol in the membrane of these T-cells and an increase in proliferation response. Of note, mice with LDLr-/- have a higher metabolism (see second link).
“Prolonged Intake of Dietary Lipids Alters Membrane Structure and T Cell Responses in LDLr−/− Mice” https://www.jimmunol.org/content/196/10/3993
“The low density lipoprotein receptor modulates the effects of hypogonadism on diet-induced obesity and related metabolic perturbations” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4076071/
7. A ketogenic diet contributes to a higher AMPK activity. AMPK helps the T-cells to survive longer and proliferate better. These memory T-cells are the ones that respond to a specific antigen which is valuable when a second infection arrives from the same virus.
“Long-term T cell fitness and proliferation is driven by AMPK-dependent regulation of reactive oxygen species.” https://pubmed.ncbi.nlm.nih.gov/33303820/
Overall it looks like a high fat, ketogenic diet seems supportive to the immune response helping in the proliferation capability and survival/efficacy in challenging environments.
There are also lots of anecdotes in various channels where people on a ketogenic diet report improvements in resistance to flu. Either they no longer get sick or the duration is shortened with less severe symptoms.
I would say, give the diet a try and take good care of yourself as we go through this COVID-19 episode.
Update 2021.06.22
Aged mCoV-A59-infected mice have increased mortality and higher systemic inflammation in the heart, adipose tissue and hypothalamus, including neutrophilia and loss of γδ T cells in lungs. Activation of ketogenesis in aged mice expands tissue protective γδ T cells, deactivates the NLRP3 inflammasome and decreases pathogenic monocytes in lungs of infected aged mice.
“Ketogenic diet restrains aging-induced exacerbation of coronavirus infection in mice” https://pubmed.ncbi.nlm.nih.gov/34151773/
9. So far these were all animal studies. The following is in vitro and in vivo human research.
We show that ketone bodies profoundly impact human T-cell responses. CD4+ , CD8+ , and regulatory T-cell capacity were markedly enhanced, and T memory cell formation was augmented. RNAseq and functional metabolic analyses revealed a fundamental immunometabolic reprogramming in response to ketones favoring mitochondrial oxidative metabolism. This confers superior respiratory reserve, cellular energy supply, and reactive oxygen species signaling.
“Very-low-carbohydrate diet enhances human T-cell immunity through immunometabolic reprogramming” https://pubmed.ncbi.nlm.nih.gov/34151532/
Update 2022.01.22
10. Monocytes-turned-macrophages are part of our immune system and present throughout our body. Acetoacetate, another ketone body produced by our body in larger numbers when on a ketogenic diet, protects the cells from metabolic disturbance due to a reduction in cellular pH.
“Acetoacetate protects macrophages from lactic acidosis-induced mitochondrial dysfunction by metabolic reprograming” https://www.nature.com/articles/s41467-021-27426-x
11. The SARS-COV-2 spike protein anti-bodies are prolonged in the body because the ketone body beta-hydroxybutyrate attaches to its free lysine. The result is that it protects the anti-body from degradation so that it remains longer in circulation. It also makes it more resistant to heat so that it is less affected by fever for example.
“Lysine β-Hydroxybutyrylation Improves Stability of COVID-19 Antibody” https://pubs.acs.org/doi/full/10.1021/acs.biomac.1c01435
Update 2022.07.06
12. More data on humans and in line with the previous quoted studies, BHB improves CD8+ T-cell functioning.
Flow cytometry and ELISA revealed elevated cytokine expression and secretion (up to + 24%) upon ketone treatment and enhanced cell lysis capacity (+ 21%). Metabolic analyses using Seahorse technology revealed upregulated mitochondrial respiratory chain activity (+ 25%), enabling both superior energy supply (+ 44%) and higher mitochondrial reactive oxygen species signaling. These beneficial effects of ketones might represent evolutionary conserved mechanisms to strengthen human immunity.
“Ketone Bodies Improve Human CD8+ Cytotoxic T-Cell Immune Response During COVID-19 Infection” https://www.frontiersin.org/articles/10.3389/fmed.2022.923502/full
13. This is more of a review paper and focused on how ketones modulate the immune barrier. It is a great and extensive paper so please do support the authors in any way you can.

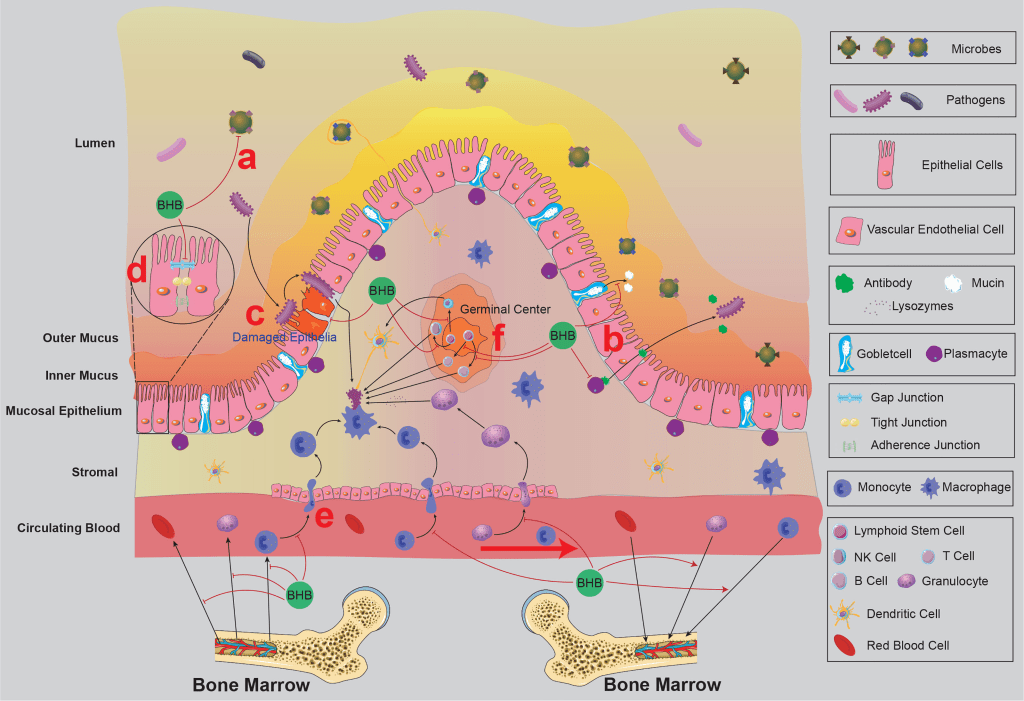
“Beta-Hydroxybutyrate: A Dual Function Molecular and Immunological Barrier Function Regulator” https://www.frontiersin.org/articles/10.3389/fimmu.2022.805881/full
update 2022.08.25
14. COVID-19 infection clearance is in part modulated by CD4+ T-cells via their IFN-γ production. Both in vitro and mouse models show an increase in IFN-γ production when BHB is added resulting in increased viral clearance and survival.
“Ketogenesis and COVID-19” https://www.nature.com/articles/s41590-022-01306-y
update 2022.08.29
This is a preprint so not peer reviewed. Check it out later to make sure it is not retracted.
15. My interpretation in point (3) seems to be correct. When your immune system must respond, it benefits from rapid replication. Being able to build up a reserve of carbon (glucose stored as glycogen) allows for a faster replication so that less time is given to the intruding pathogen to replicate. This makes the pathogen easier to isolate and exterminate.
Ketolysis is an intrinsic feature of highly functional CD8+ T effector (Teff) cells and βOHB directly increases CD8+ Teff cell IFN-γ production and cytolytic activity. Using metabolic tracers, we establish that CD8+ Teff cells preferentially use KBs over glucose to fuel the tricarboxylic acid (TCA) cycle in vitro and in vivo. KBs directly boost the respiratory capacity of CD8+ T cells and TCA cycle-dependent metabolic pathways that fuel T cell growth. Mechanistically, we find that βOHB is a major substrate for acetyl-CoA production in CD8+ T cells and regulates effector responses through effects on histone acetylation.
“Ketolysis is a metabolic driver of CD8 T cell effector function through histone acetylation” https://www.biorxiv.org/content/10.1101/2022.08.26.505402v1
update 2022.10.06
16. Furthering on the effect BHB has on CD4+ T-cells mentioned in point (14), they show how this is accomplished by fueling oxidative phosphorylation, delaying glycolysis. The authors refer to previous work that shows worse outcome when BHB production is delayed.
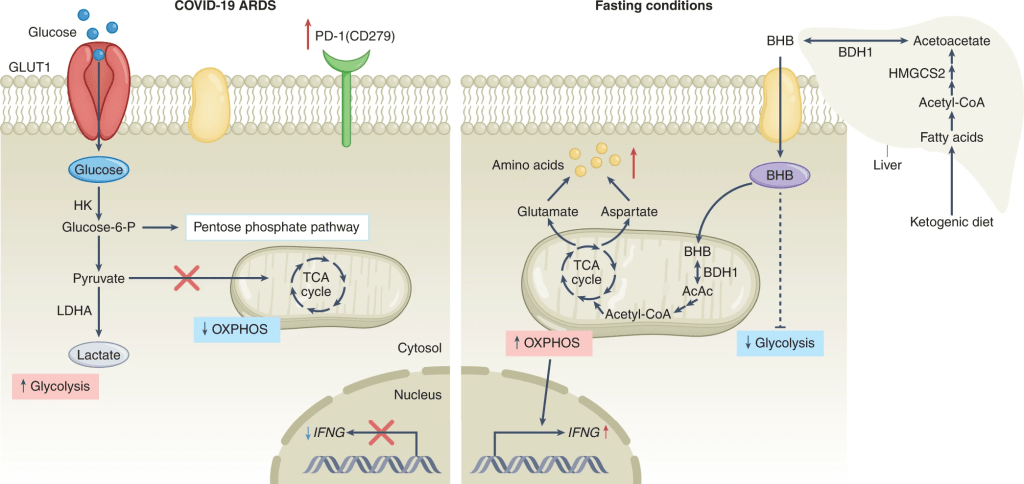
“Fasting as key tone for COVID immunity” https://www.nature.com/articles/s42255-022-00646-1
update 2023.03.23
17. In a mouse study, intermittent fasting (IF) combined with beta-hydroxybutyrate induces increase in CD4+ and CD8+ cells, paralleled by enhanced antitumor Th1 and cytotoxic responses, by enhancing their metabolic fitness.
“Intermittent Fasting induced ketogenesis inhibits mouse epithelial ovarian tumors by promoting antitumor T cell response.” https://www.cell.com/iscience/fulltext/S2589-0042(23)01916-8
update 2023.07.31
18. CD8+ T effector cell are highly dependent on their metabolism for their functioning. During a test with Listeria infection, KB availability enhanced CD8+ T cell effector function. Genetic perturbation of KB oxidation (ketolysis) in CD8+ T cells impairs effector responses to bacterial infection and cancer.
“Ketolysis drives CD8+ T cell effector function through effects on histone acetylation” https://www.cell.com/immunity/fulltext/S1074-7613(23)00314-X
update 2024.02.15
19. beta-hydroxybutyrate has 2 direct effects on colonic Treg cells. A first is to enhance their differentiation and a second is to enhance their fatty acid metabolism. This combined effect helps the mice resist ulcerative colitis.
“Exercise-induced β-hydroxybutyrate promotes Treg cell differentiation to ameliorate colitis in mice” https://faseb.onlinelibrary.wiley.com/doi/10.1096/fj.202301686RR
— THE END —
Leave a comment