Why a theory behind obesity? Isn’t it just CICO? I’ll use a simple analogy.
You find a little puddle of water so you start mopping it up but then it comes back so you do some more mopping. The puddle of water keeps coming back and bigger if you don’t do any mopping so people tell you to do more mopping and faster. You’re not doing enough to get rid of the puddle!
But if you would look at the root cause, a leaking tap, then you know you have to fix the tap and then clean up the puddle with a last mopping.
CICO is the mopping and my theory shows you how to fix the tap.
First we will have a look where the name HyProCICO comes from which sums up the whole theory in a quick easy name.
To make the information digestible I’ll first describe the theory and then go into the details to provide the backing for the theory.
I will also have a Testing section that shows some scenarios where I simply explain the findings based on my theory. Here and there supported by some reference material.
But before we get into that final Testing chapter there is first a section on the different types of leaking taps that can lead to obesity.
So the sections are:
- HyProCICO
- The Theory
- The Details
- Obesity
- Testing
HyProCICO
The name that I have given to the theory refers to the hypothalamus that does the sensing of energy and amino acids to satisfy energy needs and protect the protein in the body from being used for energy. It will do this by regulating simultaneously both Caloric Intake and Caloric Output.
The Theory
You’ll find that the theory is actually quite straightforward and doesn’t require much explaining but the ongoing debate in the (scientific) dietary communities shows this root cause is somehow missing from their view.
It will not be a complete theory. It doesn’t cover all regulation that takes place. I’m primarily looking at the most important components in relation to obesity. The hypothalamus is a central organ in this theory but this organ does so much more than the aspects touched here.
There are 2 main aspects that drive food intake. A first is the requirement for energy and a second is driven by the need for sufficient amino acids.
The brain – energy
The brain is a highly energy hungry organ. It senses how much energy is circulating in the blood via the hypothalamus. Energy includes more than just glucose. Since the brain can also run on beta-hydroxybutyrate (BHB) it measures or responds at least to the energy level through the totality of glucose and BHB but possibly also fatty acids.
The totality of energy may be the driver to stimulate hunger… However, it is possible that the level of glucose itself will drive control over what energy is freed up.
When the glucose level becomes inadequate, insulin must lower and glucagon must increase to enable the freeing of fat and conversion into ketones. The hypothalamus controls these hormones through the nervous system.
The brain – amino acids
Not only energy is required in sufficient quantity. Protein requirements also need to be met within the body to make sure cells can be maintained, misfolded or damaged protein can be cleared etc. both for the brain and the rest of the body.
As such the hypothalamus senses circulating amino acids and will stimulate feeding behavior when levels go down.
The energy sources
What happens if there is not enough energy? From the hypothalamus, nerve signals and hormones are secreted to regulate energy release from within the body storage, energy consumption by the body and external energy intake into the body.
We keep a fairly steady temperature production but it can be turned down a notch to save energy. Some people feel the urge to move or exercise a lot or can’t sit still while others don’t budge. Reducing the urge to move can also help preserve energy.
The theory will not go into the aspects of increasing or reducing energy expenditure although the regulation of it is part of why obesity establishes itself. I will briefly reference to these aspects under “The details – Energy Control”.
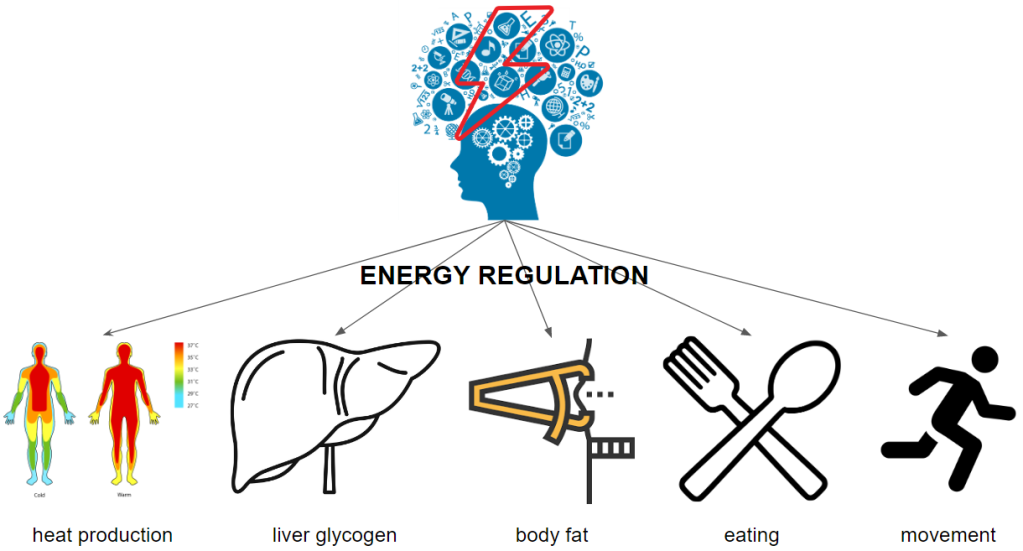
The amino acid sources
Amino acids become available through our diet by eating protein or are partially coming from recycled proteins within our body. Some of the amino acids will be lost into gluconeogenesis (GNG), no matter their source.
If levels drop below a certain threshold then feeding must be stimulated.
The danger
What if we can’t find food? What if on top of that our energy reserves are low (liver glycogen, stored fat)? As a last resort, our protein mass (skin, skeletal muscle, organs) will be broken down to continue providing energy to the brain. This is a situation that must be prevented. We have both an energy level to protect as well as an amino acid level. The brain needs both to function.
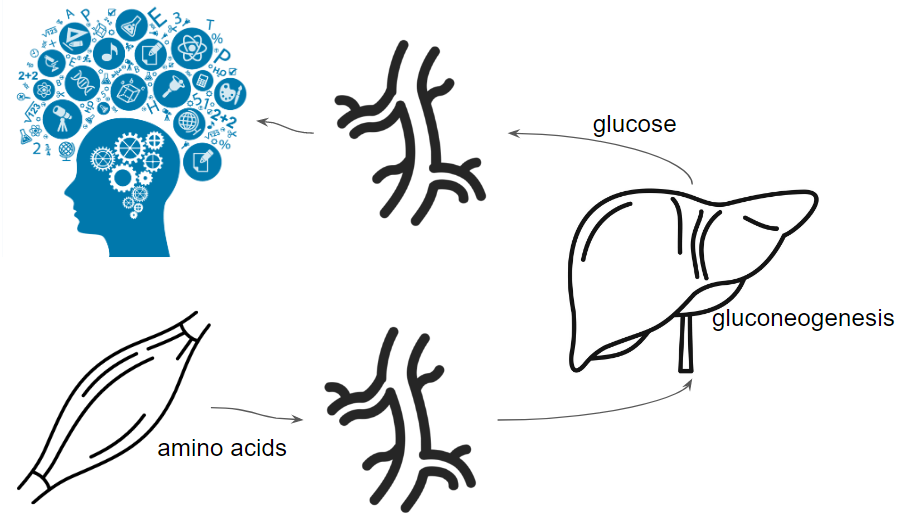
The balance
So on one hand we have the energy need of the brain that must be met and on the other hand we have the protection of protein.
If the brain senses low energy it will stimulate hunger. If the energy level is not restored, it will break down protein for energy.
The detected low energy level points out that we don’t have much reserve left so the brain better starts stimulating hunger to avoid being starved itself.
In a similar way we find that amino acids dropping in level will stimulate hunger.
What this means is that we have 2 components that both must be available in sufficient levels. We may have sufficient energy but if amino acids are low, we’ll still be stimulated to eat. Vice versa our amino acids level can be sufficient but energy may be low, also stimulating us to eat.
When there is a low level of energy sensed, the GNG process will be stimulated helping to convert circulating amino acids to glucose so low energy can lead to low amino acids. This is an important aspect when looking into obesity. It also helps explain loss of protein mass while still having sufficient fat mass when the sensing goes awkward.
The details
So much for the theory, let’s now have a look at the science behind all of it.
Energy sensing
A paper released just recently has found neurons that stimulate glucagon or inhibit glucagon release based upon the glucose level sensed.
https://www.bcm.edu/news/molecular-and-cellular-biology/glucose-sensing-neurons-blood-sugar -> “Estrogen receptor-α expressing neurons in the ventrolateral VMH regulate glucose balance” https://www.nature.com/articles/s41467-020-15982-7
Before these specific cells were identified it was already clear that the hypothalamus senses the available energy.
“Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism” https://www.nature.com/articles/emm20164
In our high-carb diet based society there is little research into the role of ketones and generally only considered as a negative element such as in diabetic ketoacidosis. Therefor we get statements like the following:
during energy deficit such as fasting specific hypothalamic glucose sensing neurons become sensitized to decreased glucose
Become sensitized to decreased glucose or equally satisfied with ketones as with glucose?
“Hypothalamic glucose sensing: making ends meet” https://www.frontiersin.org/articles/10.3389/fnsys.2014.00236/full
What I do suspect but cannot verify is that the stimulation of hunger must be dependent on the total energy but that the level of glucose specifically creates a shift to more lipolysis when its level is going down. I could not find any papers that have looked at this specific aspect.
What we can be certain about is that the brain doesn’t just look at glucose. It can also detect the level of fatty acids and ketones. The capillaries in the hypothalamus region are more ‘leaky’. Presumably to have a better and faster sensing of actual circulating levels.
“Hypothalamic Fatty Acids and Ketone Bodies Sensing and Role of FAT/CD36 in the Regulation of Food Intake” https://www.frontiersin.org/articles/10.3389/fphys.2019.01036/full
There is indirect evidence that shows ketones could be part of sensing the totality in energy. Research shows us a reduction in ghrelin when administering ketone esters. But ketones could also have a different, more direct or modulating effect on ghrelin production. Either way, BHB contributes to signaling that energy is available.
“A Ketone Ester Drink Lowers Human Ghrelin and Appetite” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5813183/
Amino acid sensing
When testing for different amino acids (l-alanine, l-arginine, l-lysine, l-proline, l-serine) they found a good response to alanine, arginine and lysine in increasing ATP.
“Amino acid sensing in hypothalamic tanycytes via umami taste receptors” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5681271/
Central administration of leucine has also been used and shown to activate hypothalamic mTOR leading to a reduction in feeding. Interesting is that leptine also stimulates mTOR in these same cells explaining the reduction in feeding behavior from leptin.
“Hypothalamic mTOR signaling regulates food intake” https://www.ncbi.nlm.nih.gov/pubmed/16690869
“Tanycyte Gene Expression Dynamics in the Regulation of Energy Homeostasis” https://www.frontiersin.org/articles/10.3389/fendo.2019.00286/full
Energy control
The hypothalamus not only senses the energy level, it also acts upon it. It controls glucagon and insulin secretion via the connected nerves.
“Hypothalamic Control of Systemic Glucose Homeostasis: The Pancreas Connection” https://www.sciencedirect.com/science/article/abs/pii/S1043276018301061
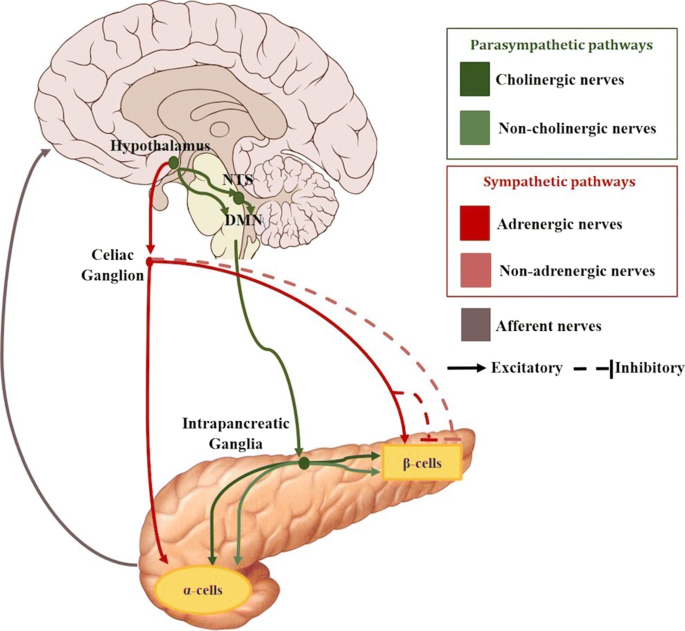
The hypothalamus can also activate the adrenals to release cytokines which will help increase lipolysis in the adipocytes.
I’ll let the next paper speak for itself:
The arcuate nucleus (ARC) of the hypothalamus contains at least two crucial populations of neurons that continuously monitor signals reflecting energy status and promote the appropriate behavioral and metabolic responses to changes in energy demand. Neurons making pro-opiomelanocortin (POMC) decrease food intake and increase energy expenditure through activation of G protein-coupled melanocortin receptors (MCR) via the release of a-melanocyte-stimulating hormone (aMSH). Until recently, the prevailing idea was that the neighboring neurons expressing the orexigenic neuropeptides, agouti-related protein (AgRP) and neuropeptide Y (NPY) (NPY/AgRP neurons) increased feeding and decrease energy expenditure primarily by opposing the anorexigenic/catabolic actions of the POMC through both the competitive inhibition of melanocortin tone at the postsynaptic level and via directed inhibition of POMC firing rate (Fig. 1)
You see here how energy shortage is reacted on by increasing feeding and decrease energy expenditure and energy abundance lowers food intake and increases energy expenditure.
“The hypothalamic arcuate nucleus and the control of peripheral substrates” https://www.sciencedirect.com/science/article/abs/pii/S1521690X14000505
Now you can understand why weight loss strategies that reduce food intake and demand increase in energy expenditure through activity are completely contradictory to how the body wants to regulates itself ! We think it is all about the energy we eat while it is about the energy that the brain senses. Feeding and decreasing energy expenditure belong to each other, satiety and increased energy expenditure belong to each other.
The following picture is created to show the role of leptin but it gives a good overview of the hypothalamus and several of its structural elements. Mainly the tanycytes which are the sensors, their presence close to the fenestrated capillaries and how tanycytes affects NPY and POMC.

Hunger stimulation
In a mouse test they injected BHB to make it directly available to the hypothalamus but they noted an increase in feeding behavior. That goes against my theory because we’re injecting even more energy than what already circulates so if anything they should be less hungry.
This is a good case of why research leads to wrong conclusions and thus creates confusion. Luckily though, they investigated thoroughly and even confirmed in their discussion that there was a downregulation of glucose AND MCT transporters in the blood-brain-barrier (through which BHB can get into the brain). With lower energy sensed by the hypothalamus due to lowered glucose and BHB you stimulate feeding behavior. Just like the theory predicts.
It would be interesting to find out why such injection has lead to reduction in MCT.
“Hypothalamic sensing of ketone bodies after prolonged cerebral exposure leads to metabolic control dysregulation” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052612/
Ghrelin is a hormone secreted in the stomach and said to induce hunger. Research shows us that it depends on NPY and AgRP which are secreted by the hypothalamus. At least one of these 2 elements is needed for ghrelin’s effect.
“Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein.” https://www.ncbi.nlm.nih.gov/pubmed/14962995
Ghrelin is highest before a meal and lowest after. As such it is a hormone that signals digestion of food. By signaling that there is nothing in the digestion, through raising ghrelin levels, it gives the hypothalamus a sense that there is no incoming energy or amino acids. And this also means that there is room to stimulate dietary intake.
A mouse knockout model of the ghrelin receptor in the hypothalamus leads to increase in energy expenditure and ablation of obesity. This simulates a signal that food is being digested thus energy and amino acids will be coming in so it increases energy expenditure.
“Neuronal Deletion of Ghrelin Receptor Almost Completely Prevents Diet-Induced Obesity.” https://www.ncbi.nlm.nih.gov/pubmed/27207529
And this means we get a similar effect when we reduce the ghrelin hormone instead of the receptor in the hypothalamus. It comes down to the same thing.
“Absence of ghrelin protects against early-onset obesity.” https://www.ncbi.nlm.nih.gov/pubmed/16322795
Obesity
The theory shows a mechanism in which there is a well balanced sensing and regulation. As long as the total energy is OK and amino acids are OK then there is no issue.
When it comes to energy, in order to stimulate hunger and create a surplus in body weight, we must have a chronic situation where there is sufficient energy available but 1) the energy in the circulation is ‘under representing’ what is available OR 2) there is a problem in the sensing or representation of the circulating energy in the hypothalamus.
- For various reasons it is possible that energy is not released sufficiently so there is a reduced level of energy in the blood circulation.
- The sensing is a never-ending process depending on the continuous passage of blood. Any reduction or increase in blood flow can drive less or more energy to the hypothalamus. Any issue in processing that energy level in the hypothalamus cells will lead to a wrong correction.
When the sensing detects low energy, it will stimulate feeding. No matter how much energy is available in storage.
When it comes to amino acids, a low sensed level can lead to a surplus intake if the dietary protein are low while the energetic value of the food is high. Food with low protein content will not be able to raise the circulating amino acid levels enough so more food needs to be eaten to come up with a sufficient level of amino acids.
A paper from Kevin Hall indicates this by having compared ultra-processed food with unprocessed food. Free intake of food resulted in a perfect match of protein intake (490 kcal/d) between the 2 groups. With energy sufficiently available, it was the protein that drove the intake in this study.
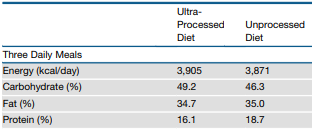
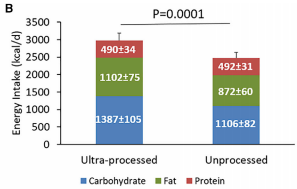
“Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake.” https://www.ncbi.nlm.nih.gov/pubmed/31105044
So what are some of the various cases apart from protein dilution in the meal?
Fructose
I’ve already covered the detrimental roles that fructose play in health and pointed out its link with obesity but let’s focus in on its specific action on the hypothalamus.
As highlighted earlier, the signaling works through the activation of mTOR for which sufficient ATP needs to be available. Glucose, BHB, fatty acid all ensures sufficient ATP. Fructose however depletes the ATP in the cells when it gets metabolized. That will lead to activation of AMPK which is kind of the opposite of mTOR.
The reduction of ATP causes a signal of reduced energy availability. The body responds by increasing hunger and lowering energy expenditure.
“Differential effects of central fructose and glucose on hypothalamic malonyl–CoA and food intake” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2579345/
Fructose can be taken up from the diet but it can also be produced endogenously. When fructose is metabolized, it leads to an increase uric acid. Uric acid itself activates aldose reductase which is responsible for converting glucose to fructose so fructose has a positive feedback loop whereby fructose ingestion and metabolism further increases fructose production and metabolism.
“Uric acid activates aldose reductase and the polyol pathway for endogenous fructose
and fat production causing development of fatty liver in rats” https://www.ncbi.nlm.nih.gov/pubmed/30651350
Insulin is also involved in the energy signaling and regulation by the hypothalamus. Insulin is a known activator of mTOR so it will stimulate the sense of energy excess leading to satiety and energy expenditure. The cells in the hypothalamus express insulin receptors. It is possible that the metabolism of fructose within these same hypothalamus cells creates a similar insulin resistance as happens in the liver.
“Insulin controls food intake and energy balance via NPY neurons” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5444095/
Pointing out further the effect of fructose metabolism, when fructose is absorbed in liquid form and in high enough quantity and frequently, it will lead to insulin resistance causing high secretion of insulin for prolonged time. This appearance of insulin resistance can be seen as an indication that fructose is metabolized in the body and is thus able to reach the brain where its metabolic effect is indicated above.
“Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial.” https://www.ncbi.nlm.nih.gov/pubmed/22933433
Hypoglycemia
Eating frequent high-insulin-secreting meals may lead to periods of hypoglycemia. During this hypoglycemia hunger will rise stimulating more eating because energy (glucose) in the circulation is low.
“Hyperinsulinemic Hypoglycemia – The Molecular Mechanisms” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2579345/
There are various drugs that can lead to hypoglycemia, mainly glucose-controlling drugs for type 2 diabetes or a too strong injection of insulin for a type 1 diabetic.
“Hypos and Controlling Hunger” https://www.diabetes.co.uk/hypos-and-controlling-hunger.html
2DG
2-deoxy-D-glucose or 2DG interferes with glucose metabolism and drives hunger.
“Hunger in humans induced by 2-deoxy-D-glucose: glucoprivic control of taste preference and food intake.” https://www.ncbi.nlm.nih.gov/pubmed/929188
As we saw before under fructose, ATP depletion caused activation of AMPK. 2DG, by hindering glucose metabolism in the hypothalamus also causes a reduction in ATP with a resulting increase in AMPK, stimulating an increase in glucagon and corticosterones.
“Role of Hypothalamic Adenosine 5′-Monophosphate-Activated Protein Kinase in the Impaired Counterregulatory Response Induced by Repetitive Neuroglucopenia” https://academic.oup.com/endo/article/148/3/1367/2502178
Antidepressants
The long term usage of antidepressants is associated with weight gain. When I bumped into this I first wanted to check if they are feeling more hungry. And indeed, it is even used as a combo in underweight elderly to handle their depression and weight at the same time.
“Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study” https://www.bmj.com/content/361/bmj.k1951
“Carbohydrate craving and increased appetite associated with antidepressant therapy.” https://www.ncbi.nlm.nih.gov/pubmed/3197015
“Antidepressant use in underweight older adults.” https://www.ncbi.nlm.nih.gov/pubmed/23229075
So could there be any influence of antidepressants on the glucose sensing in the hypothalamus? It turns out that the drug citalopram, an SSRI antidepressant, reduces the blood flow in the hypothalamus.
“Serotonin modulation of cerebral blood flow measured with positron emission tomography (PET) in humans.” https://www.ncbi.nlm.nih.gov/pubmed/15668991
Do we find citalopram associated with weight gain?
Celexa (citalopram) has been associated with slight weight gain, but it’s thought that the drug itself doesn’t cause this effect. Rather, the weight increase is likely due to improved appetite from taking the drug. A better appetite can cause you to eat more, leading to increased body weight.
https://www.healthline.com/health/depression/celexa-weight-gain#antidepressants-and-weightgain
I guess we now know where that appetite comes from… Sensing a reduction in available energy due to the reduced blood supply.
Citalopram (−0.1 to +7.1 kg 42,59,61) is associated with the greatest amount of weight gain
“Medications that cause weight gain and alternatives in Canada: a narrative review” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6109660/
Melatonin
Because I remember from hearsay that lack of sleep can cause weight gain I thought this would be interesting to check out. The story is more complex here because it is specific to sleep and temperature regulation is important since we can’t simply put on an extra cover when we are cold.
A study in rats does show melatonin induces a reduction in blood flow in all areas including in the hypothalamus. But is it representative for humans?
Such a brain wide reduction in blood flow would also result in a reduction of oxygen supply. In addition it also lowers energy signaling from all other hormones such as leptin, ghrelin, insulin etc.. Interesting…

“Reduction of regional cerebral blood flow by melatonin in young rats.” https://www.ncbi.nlm.nih.gov/pubmed/7670001
We do find, also in rats, that melatonin is inversely related to insulin production.
“Melatonin and pancreatic islets: interrelationships between melatonin, insulin and glucagon.” https://www.ncbi.nlm.nih.gov/pubmed/23535335/
Sleep deprived young adults go for a bigger breakfast. Is the weight control due to the size of our breakfast alone or an extra factor?
“Acute sleep deprivation increases portion size and affects food choice in young men” https://www.sciencedirect.com/science/article/pii/S0306453013000176
Temperature
Melatonin is produced during the whole night and during our sleep the body temperature drops. How is this temperature regulated at night?

During our sleep, temperature is diverted from the core to the skin through vasodilation. This probably evolved in our evolution that way to protect us from too cold ambient temperatures with death or frost bite from cold during sleep.
“Sleep and thermoregulation” https://www.sciencedirect.com/science/article/pii/S2468867319301804
We find that melatonin increases BAT and beige activity which are known to create heat by uncoupled metabolism which means consuming energy just for the sake of producing warmth. It is possible that melatonin acts directly on the fat cells so it doesn’t work through the hypothalamus.
“Melatonin increases brown adipose tissue mass and function in Zücker diabetic fatty rats: implications for obesity control” https://onlinelibrary.wiley.com/doi/abs/10.1111/jpi.12472
“Melatonin multiple effects on brown adipose tissue molecular machinery.” https://www.ncbi.nlm.nih.gov/pubmed/30597601
“Melatonin Increases Brown Adipose Tissue Volume and Activity in Patients With Melatonin Deficiency: A Proof-of-Concept Study” https://diabetes.diabetesjournals.org/content/68/5/947
This is perfect to keep warm during the night and would explain the reduction in brain blood flow to simulate energy shortage, thereby releasing more energy which then can be used for the heat generation.
The hypothalamus can also induce thermogenesis but this is part of energy expenditure and falls under sensing sufficient energy and is also stimulated by cold sensation signals (like a cold shower).
The level to which our temperature can drop will make a difference in how much energy needs to be spent to keep us warm.
What we see in obese subjects, a high energy evening meal causes less weight reduction versus an isocaloric daily intake where the largest portion is taken during the morning.
“High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women.” https://www.ncbi.nlm.nih.gov/pubmed/23512957
We also see that the higher caloric content during the evening meal causes a smaller drop in night time temperature (in healthy subjects).
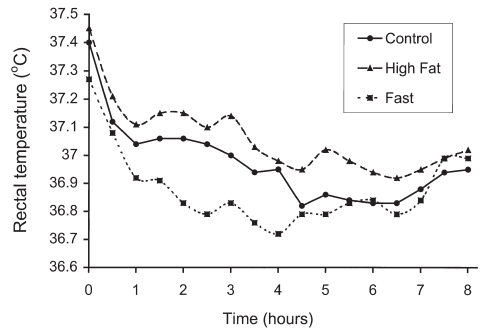
“Energy content of the evening meal alters nocturnal body temperature but not sleep.” https://www.ncbi.nlm.nih.gov/pubmed/10627057
Testing
I’m trying to look at a diverse list of scenarios to see if I can explain the outcome using the theory. In such a way you’ll be able to understand how I see the model working and allows you to refute or ask questions. My theory does need validation.
Glycogen Storage Disease type III (GSD3)
A first example is GSD3 where they report a prevalence of enlarged liver (hepatomegaly) of 98%. This disease has a problem with breaking down glycogen into glucose to release it into the circulation. It also shows us that there is not really a fixed ceiling for storing glucose in the liver. The liver adapts growing larger to store more.
The patients are intolerant to fasting. They can generate ketones but their glucose levels are too low (because they cannot free it up fast enough from the liver) despite the ketones they may produce.
One of their symptoms is (chronic) hunger. With their reduced available glucose, it could be beneficial if they are on a high fat diet to generate more ketones and as such provide the necessary circulating energy.
“Glycogen Storage Disease Type III” https://rarediseases.org/rare-diseases/forbes-disease/
“The long-term outcome of patients with glycogen storage diseases” https://link.springer.com/article/10.1007%2FBF01799498
“Modified Atkins ketogenic diet improves heart and skeletal muscle function in glycogen storage disease type III.” https://www.ncbi.nlm.nih.gov/pubmed/31309177/
Inuit
Linked to GSD3 but actually the opposite we find in Inuits. They have a mutation that reduces their ketone production capacity. Where GSD3 cannot provide enough glucose, we find in the Inuits affected by this mutation that they cannot provide sufficient ketones.
“A Selective Sweep on a Deleterious Mutation in CPT1A in Arctic Populations” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4225582/
One of the hypothesis for this mutation is that this mutation is beneficial for the production of heath. As an elderly has been found saying:
When one eats seal, you are full all day. When you eat packaged foods, two hours later you get cold. If [you] eat Inuit food, you stay warm.
“The Changing Landscape of Arctic Traditional Food” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2944111/
Not only does the high protein allows them to maintain glucose level but in addition is the fat helping them with keeping warm in the arctic climate.
I couldn’t find any clear references but traditional eskimos are said to have enlarged livers and eat a high level of protein. If this is correct, it would fit the theory in such a way that there must be an increased capacity to produce glucose for a longer period of time throughout fasting in compensation for a reduced ketones production capacity.
If anyone can contribute to finding good references towards the protein intake and the liver size then I would be very grateful.
“Extreme Nutrition: The Diet of Eskimos” https://www.drmcdougall.com/misc/2015nl/apr/eskimos.htm
Their traditional food does seem to provide a large amount of protein. I see numbers around 133~166gr of protein for adult males (20-60y) while they have an average height of around 165 cm. That would support a high protein consumption.
“Dietary nutrient profiles of Canadian Baffin Island Inuit differ by food source, season, and age.” https://www.ncbi.nlm.nih.gov/pubmed/8557942
“Height, Weight, and Growth of Alaskan Eskimos” https://jamanetwork.com/journals/jamapediatrics/article-abstract/502131
High Protein
A study tested the effects of hunger, appetite and weight loss in 2 different groups. A first group on a low carb high protein diet (30%p; 4%c; 66%f) and another group on a medium carb high protein diet (30%p; 35%c; 35%f). They were fed ad libitum. To understand the results make sure you have also read my article on gluconeogenesis being a supply driven process.
What happens here is that the high protein intake helps to replenish the glucose level in the liver for both groups. The difference however is that the low carb group has lower meal-triggered insulin release. This allows the low carb group to release more fat for energy which leads to ketone production.
The medium carb group has a very high insulin secretion due to being combined with high protein. This is how incretins work out (check out the video under “Regulation”). This raises the excursions into hypoglycemia post absorption. In such a phase the hypothalamus may react with hunger stimulation.
“Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum.” https://www.ncbi.nlm.nih.gov/pubmed/18175736
Obesity-related high leptin and low ghrelin levels
We find sufficiently high leptin and sufficiently low ghrelin levels in obese people.
“The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review.” https://www.ncbi.nlm.nih.gov/pubmed/17212793
Both low ghrelin and high leptin should signal to the hypothalamus that there is abundance of energy so it should lead to a reduction in appetite and increase energy expenditure.
Active research in this field has defined the term leptin resistance. One of the mechanisms could be that leptin has a reduced capability to cross the blood brain barrier (BBB) and as such cannot provide its signaling. This has been shown in mice by leptin administration directly in the brain, bypassing the BBB. What causes this issue in crossing the BBB is under investigation. There are also thoughts regarding disturbed signaling because the hypothalamus could get inflammed when metabolising fructose due to the stress of low ATP availability.
“Leptin Resistance and the Neuro-Adipose Connection” https://www.frontiersin.org/articles/10.3389/fendo.2017.00045/full
“Diet-induced obese mice develop peripheral, but not central, resistance to leptin.” https://www.jci.org/articles/view/119171
When it comes to ghrelin, it is probably a non-factor. Meaning that high ghrelin can induce hunger but it does not mean that low ghrelin induces satiety nor that high ghrelin is needed to induce hunger. Activation of AMPK is already sufficient to release NPY so although low ghrelin may signal digestion is going on, if AMPK is activated for some reason then hunger is stimulated.
Obese insulin sensitive and obese insulin resistant
There are obese people who develop insulin resistance as you would expect from high fructose consumption. Yet there are also those who remain sensitive.
I’m missing quite some data on these people but in the following study something caught my eye. The obese sensitive people have significant lower fasting triglycerides. What this may mean is that the fat which is produced in the liver from fructose is quicker cleared and moved out of the liver. This would prevent NAFLD and insulin resistance.
They may have a quicker response to the fructose induced lowering of insulin and increase in glucagon. By quicker releasing the fat from the liver there is less chance of building it up to cause restance.
The fat however is created and needs to be stored elsewhere.
“Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: role in prediction of incident pre-diabetes in a longitudinal biracial cohort” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5574414/
Perinatal protein restriction
The development of a fetus during a period with inadequate maternal protein consumption has consequences for the offspring. This has been tested in Sprague-Dawley rats. What is interesting about this experiment is that the offspring rats had an increase in hunger, consuming more calories, yet at a lower body weight which the authors suspect is due to increased energy expenditure. This is a very big difference in energy expenditure. It is not eat more and weigh the same, but eat more and weigh less.
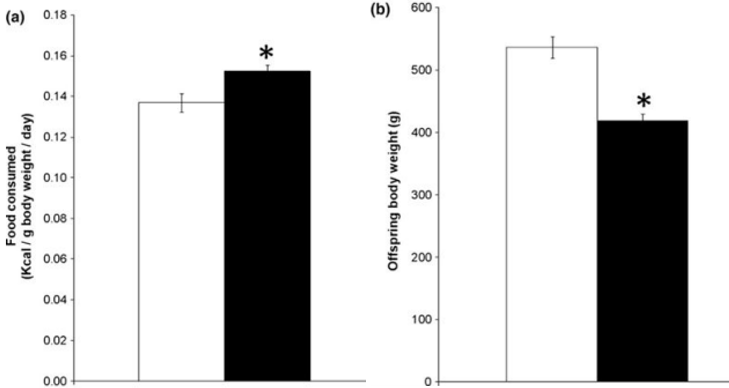
Qasem RJ, Li J, Tang HM, Pontiggia L, D’mello AP. Maternal protein restriction during pregnancy and lactation alters central leptin signalling, increases food intake, and decreases bone mass in 1 year old rat offspring. Clin Exp Pharmacol Physiol. 2016;43(4):494‐502. doi:10.1111/1440-1681.12545 https://pubmed.ncbi.nlm.nih.gov/26763577/
So what is going on? The following study gives us a glimps of what may have taken place. They looked at hypothalamic cells in the fetus of maternally protein restricted rats. Most of the upregulated genes are involved in the mitochondrial complex. I suspect this is not only in the hypothalamus but system wide and is done to increase mitochondrial mass with the purpose of increasing the protein protection by enhancing fatty acid metabolism.
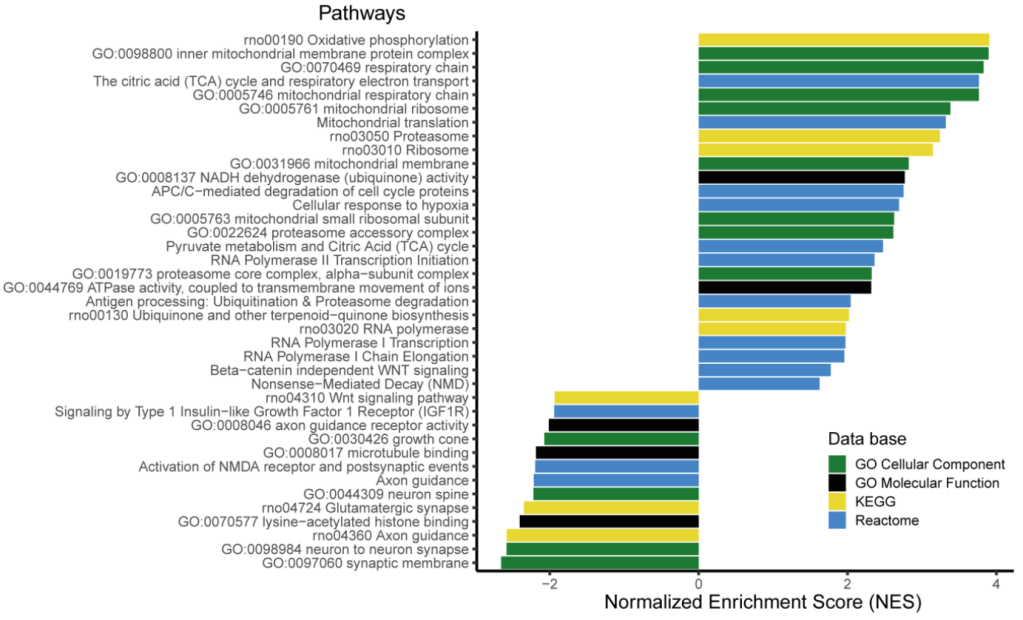
Frapin M, Guignard S, Meistermann D, et al. Maternal Protein Restriction in Rats Alters the Expression of Genes Involved in Mitochondrial Metabolism and Epitranscriptomics in Fetal Hypothalamus. Nutrients. 2020;12(5):E1464. Published 2020 May 19. doi:10.3390/nu12051464 https://pubmed.ncbi.nlm.nih.gov/32438566/
Further changes noted in the offsprings is an enhanced gluconeogenesis capability. Improving the ability of the liver to increase glycogen storage and maintain glucose output is another way to protect protein from serving as a glucose substrate.
Liu X, Wang J, Gao L, Jiao Y, Liu C. Maternal Protein Restriction Induces Alterations in Hepatic Unfolded Protein Response-Related Molecules in Adult Rat Offspring. Front Endocrinol (Lausanne). 2018;9:676. Published 2018 Nov 20. doi:10.3389/fendo.2018.00676 https://www.frontiersin.org/articles/10.3389/fendo.2018.00676/full
Transition into a ketogenic diet
A very recent study, also from Kevin Hall, comparing high carb with low carb ad lib intake shows again the need to meet sufficient amino acid levels. This time however there was not an equal intake of dietary protein so what happened?
The previous study from Hall (see “Obesity”) had a different distribution in macronutrients. Here the much higher carb offers more protection from protein catabolism by providing a large amount of glucose and stimulation of insulin. Insulin protects the muscle from breakdown and the high glucose makes sure the liver can supply a steady stream of glucose to satisfy the needs of the brain.
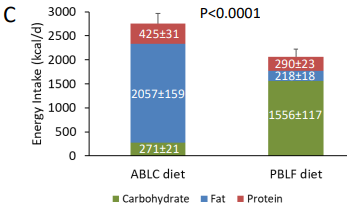
OK but why did the low carb diet led to such a high intake? By taking out carbs from the diet, the liver glycogen lowers. This lowers glucose availability. The brain will react by lowering insulin and increasing glucagon. This will lead to a higher level of GNG whereby also the circulating amino acids are converted to glucose.
BHB can compensate for the lower glucose but the production of BHB is not immediately increased. To cover this transition until BHB production is high enough, protein need to fill in as a source of energy (glucose) so there is a temporary need to increase protein intake to help supply the circulating glucose and circulating amino acids.
The body does not want to give up its own protein so it will increase hunger feeling. This will lead to an increase in food intake whereby the food intake will directly cover the glucose requirements. The food contains little carbs so the protein in the food will for a part be converted to glucose while at the same time the dietary protein also have to serve as a supply to maintain amino acid levels.
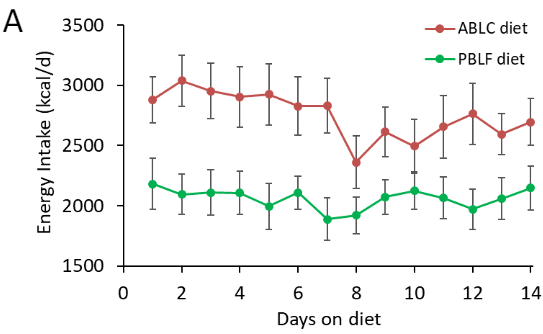
In the first week we see an immediate jump up in dietary intake. During the first days BHB production is too low to help compensate for the drop in glucose. As BHB ramps up throughout the week we see the intake reduce. In the second week we see a marked lower intake versus the first week now that BHB has reached a meaningful level.
There is still a difference in caloric intake because the protein in the diet are not sufficient to keep the circulating amino acids up. GNG is high for the low carb diet and there is very little glucose from the diet. A longer study is required to see how this further evolves. This study has helped us view what happens during the first 2 weeks when transitioning into a low carb diet.
Preprint reference: “A plant-based, low-fat diet decreases ad libitum energy intake compared to an animal-based, ketogenic diet: An inpatient randomized controlled trial” https://osf.io/preprints/nutrixiv/rdjfb/
Once the transition period is over, it will be easier for the body to obtain energy from BHB through fat metabolism and glucose through the GNG process. There is a system wide adaptation whereby the brain will start making ketones, the skeletal muscle will use more fat for energy etc..
“Hypothalamic Fatty Acids and Ketone Bodies Sensing and Role of FAT/CD36 in the Regulation of Food Intake” https://www.frontiersin.org/articles/10.3389/fphys.2019.01036/full
The whole system has to adapt to rearrange how it provides sufficient energy to the brain and thereby finds a new equilibrium to spare amino acids.
To further support the “transition period” with more evidence, the following study shows a longer trial of 30 days whereby we see an accelerating fat loss after 15 days. It was also conducted by Kevin Hall et all.
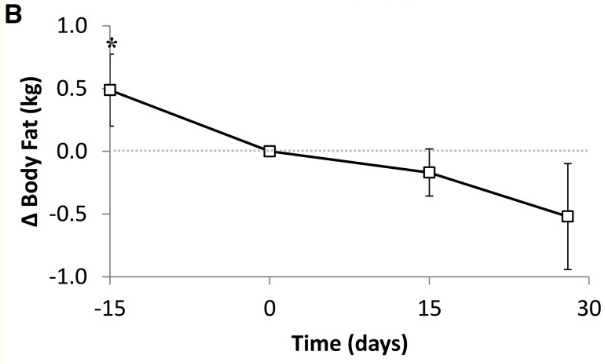
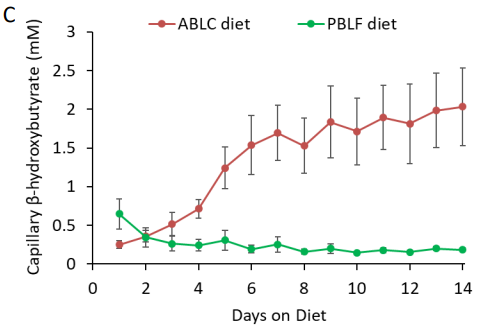
It was also noted that there was increase urinary nitrogen. As I explained, the lack of sufficient compensation by BHB during the transition will result in the breakdown of protein.
Urinary nitrogen excretion increased by 1.5 ± 0.4 g/d (Table 3; P = 0.0008) during the KD phase and indicated significantly increased protein utilization. The time course of the changes in urinary nitrogen excretion showed that the increased protein utilization occurred within the first week of the KD and persisted until day 11 (not shown).
“Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4962163/
Further support for the transition effect comes from a study where they tested the low carb effects of exercise across a very short duration. Via phenylalanine they found a greater oxidation of the amino acid showing that if you are not sufficiently transitioned, low liver glycogen will result in low glucose and therefor a greater amino acid conversion to glucose via GNG.
“Low-Carbohydrate Training Increases Protein Requirements of Endurance Athletes.” https://www.ncbi.nlm.nih.gov/pubmed/31083047
And there are a few more longer run studies that show the transition effect and how this is resolved after roughly the 2 week mark. Both noted a greater proteolysis in the first 2 weeks.
“The Human Metabolic Response to Chronic Ketosis Without Caloric Restriction: Physical and Biochemical Adaptation” https://pubmed.ncbi.nlm.nih.gov/6865775/
“Protein Sparing During Treatment of Obesity: Ketogenic Versus Nonketogenic Very Low Calorie Diet” https://pubmed.ncbi.nlm.nih.gov/1556948/
Forced overfeeding
In an experiment to overfeed people we see that calories do matter to gain weight. But what the referenced study did was gradually increase overfeeding (20%, 40%, 60%) with each time a period of ad lib food intake to satiety. In the ad lib period after the 60%, the subjects naturally started to eat less calories than at baseline.
None of their allowed drinks contained any liquid sugar or fructose allowing for the automatic regulation of energy intake and expenditure.
Finally the authors conclude:
regulation must be dominated by hypothalamic modulation of energy intake. This result supports present conclusions from genetic studies in which all known causes of human
obesity are related to defects in the regulation of appetite.
Siervo M, Frühbeck G, Dixon A, et al. Efficiency of autoregulatory homeostatic responses to imposed caloric excess in lean men [published correction appears in Am J Physiol Endocrinol Metab. 2008 Apr;294(4):E808]. Am J Physiol Endocrinol Metab. 2008;294(2):E416‐E424. doi:10.1152/ajpendo.00573.2007 https://pubmed.ncbi.nlm.nih.gov/18042669/
Migraine (added 1 dec 2020)
Today (1 dec 2020) I bumped against a post on Twitter explaining why you can get hungry when you have a migraine. Because of this theory I immediately suspected an impact on hypothalamic blood flow so a great case to test the theory.
As it turns out, this year a paper came out showing a reduction in blood flow in the hypothalamus. It perfectly demonstrates how the hypothalamus is the central organ that detects energy and responds accordingly.
Why the blood flow decreases is unfortunately not revealed.
Our results reflect that immediately prior to a migraine headache, resting regional cerebral blood flow decreases in the lateral hypothalamus. In addition, resting functional connectivity strength decreased between the lateral hypothalamus and important regions of the pain processing pathway, such as the midbrain periaqueductal gray, dorsal pons, rostral ventromedial medulla and cingulate cortex, only during this critical period before a migraine headache.
“Altered regional cerebral blood flow and hypothalamic connectivity immediately prior to a migraine headache” https://journals.sagepub.com/doi/abs/10.1177/0333102420911623
Lauric Acid (added January 2021)
There was a study done in rats to find out the effect of lauric acid, a medium-chain fatty acid. They wanted to study the anti-obesogenic properties of lauric acid. They noticed a modulation of NPY and AGRP in the hypothalamus showing there is a reduction in hunger stimulation.
The mRNA expression levels of the anorexic neuropeptide POMC in the hypothalamus between the LT group and the other groups were not different, while the gene expression levels of the orexigenic neuropeptides NPY and AGRP decreased significantly in the LT group.
“Lauric Triglyceride Ameliorates High-Fat-Diet-Induced Obesity in Rats by Reducing Lipogenesis and Increasing Lipolysis and β-Oxidation.” https://pubmed.ncbi.nlm.nih.gov/33433211
Vagus Nerve (Added January 2021)
The hypothalamus, based on what it detects will control the vagus nerve to control the pancreas in releasing glucagon and insulin. The following experiment shows that when stimulating the vagus nerve, it will lead to a higher metabolism and more weight loss in an isocaloric setting.
“Vagus Nerve Stimulation Reduces Body Weight and Fat Mass in Rats” https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0044813
The stimulation of the vagus nerve also seems responsible for the browning of white adipose tissue. This makes the adipose tissue more active and in part will result into a higher energy expenditure.
“Electric stimulation of ears accelerates body weight loss mediated by high-fat to low-fat diet switch accompanied by increased white adipose tissue browning in C57BL/6 J mice” https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/s12906-018-2388-1
Liraglutide (Added May 2021)
This drug is injected and successfully treats diabetes and causes weight loss. What does it do? It’s a GLP-1 receptor agonist, basically it mimicks GLP-1 that is normally released as food comes in and tells us that we have eaten enough.
“Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined” https://www.nejm.org/doi/full/10.1056/NEJMoa2028198
We see that it reduces the response to highly desirable food so the mental desire for food is influenced by physical factors with signals passing through the hypothalamus.
“GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial” https://pubmed.ncbi.nlm.nih.gov/26831302/
How it achieves the increase in weight reduction is through increasing metabolism by stimulating T4 secretion.
“Chronic intrahypothalamic rather than subcutaneous liraglutide treatment reduces body weight gain and stimulates the melanocortin receptor system” https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5550563/
Bile Acid (Added May 2021)
The evidence is now quite broad but let me add one final element. Central administration of bile acid in the brain, presumably signaling incoming food and more specifically fat, signals energy and is responded to by stimulating the sympathetic nervous system. This increases the metabolic rate through various ways leading to reduced weight accumulation, up to weight loss itself.
“Hypothalamic bile acid-TGR5 signaling protects from obesity” https://pubmed.ncbi.nlm.nih.gov/33887197/
Ventromedial hypothalamus (VMH) manipulation (Added June 2021)
The VMH is the center which stimulates hunger. By ways of a lesion, in rats they are able to cause insulin to secrete 60%~280%-fold. That is enormous.
“Correlation between Hyperinsulinemia and Hyperphagia in Rats with Ventromedial Hypothalamic Lesions” https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1748-1716.1972.tb05152.x
Furthermore, the VMH responds to the thyroid hormone via the thyroid hormone receptor (THR). By knocking out the receptor the animals start to overeat.
“Thyroid Hormone Receptor Beta in the Ventromedial Hypothalamus Is Essential for the Physiological Regulation of Food Intake and Body Weight” https://www.cell.com/cell-reports/supplemental/S2211-1247(17)30723-4
Pot-smoking and gastric bypass versus gastrectomy (Added September 2021)
The hypothalamus has a receptor for endocannabinoids (CB1). Both surgical methods to reduce the amount of food intake are met with different effects on resting metabolic rate because of a difference in endocannabinoid levels. Low levels of cannabinoids stimulate energy expenditure via brown fat activation while higher levels reduce it. This brown fat activation is triggered via sympathetic nervous stimulation, done by the hypothalamus.
This same receptor CB1 is responsible for the effect of feeling hungry when smoking cannabis.
“Endocannabinoid Receptor-1 and Sympathetic Nervous System Mediate the Beneficial Metabolic Effects of Gastric Bypass” https://www.cell.com/cell-reports/fulltext/S2211-1247(20)31259-6?sf239289978=1
“The role of endocannabinoids in the hypothalamic regulation of visceral function” https://www.sciencedirect.com/science/article/abs/pii/S0952327801903539
“Endocannabinoids in the regulation of appetite and body weight” https://pubmed.ncbi.nlm.nih.gov/16148436/
Cannabis also affects the secretion of gut hormone GLP-1, leading to a lower insulin response. That insulin response is directly affected by GLP-1 levels but the hypothalamus also responds to GLP-1 as we have seen earlier under liraglutide.
“Effects of oral, smoked, and vaporized cannabis on endocrine pathways related to appetite and metabolism: a randomized, double-blind, placebo-controlled, human laboratory study” https://www.nature.com/articles/s41398-020-0756-3
Finally
As you can see, the hypothalamus is central to the regulation and it acts based upon all the different inputs that tell us something about the energetic status of the whole body.
I hope it is clear with this article that obesity is not simply a matter of calories. Yes a calorie is a calorie and under controlled equal caloric feeding you may not gain weight but such controlled feeding is not our natural world. However, there are even rodent lab models that you can even give a lower caloric content while still gaining weight versus healthy controls.
When people get obese, we need to think in terms of energy and amino acid sensing. It’s the sensing that needs to be fixed, not how much we actually have available. We have no long lasting will power over this sensing, we can fight it but eventually succumb to the automated regulation.
When the brain signals hunger and lowers metabolism… eating less and moving more is completely opposite of what the body wants to achieve and only further aggravates the signal if we don’t fix the underlaying problem.
We need to work with the system, not against it:
- Protect your protein by having a sufficient supply of circulating amino acids
- Avoid any other issue outlined above that would interfere with the correct sensing of energy or would interfere with releasing sufficient energy from the storage places
Take care of these problems and the body will auto-regulate itself towards a more lean and active individual.
For most people, taking out liquid fructose (and alcohol) and sugar in general will solve much of the problem so consider this number 1 on the priority list.
Note: No doubt that there are other causes that can lead to obesity but they likely will all show somehow to influence the described mechanism and may not always be fixable when they cannot be adapted for by lifestyle changes.
— THE END —
Leave a comment